In chemistry the mole is a fundamental unit in the Système International d'Unités, the SI system, and it is used to measure the amount of substance. This quantity is sometimes referred to as the chemical amount. In Latin mole means a 'massive heap' of material. It is convenient to think of a chemical mole as such.
- One Mole Of A Substance Contains How Many Atoms Or Molecules
- One Mole Of A Substance Contains Dash Atoms Or Molecules
- One Mole Of A Substance Contains
- One Mole Of A Substance Contains Atoms Or Molecules
The SI base unit for amount of substance is the mole. 1 mole is equal to 6.0221415E+23 molecule. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between moles and molecules.
Visualizing a mole as a pile of particles, however, is just one way to understand this concept. A sample of a substance has a mass, volume (generally used with gases), and number of particles that is proportional to the chemical amount (measured in moles) of the sample. For example, one mole of oxygen gas (O 2 ) occupies a volume of 22.4 L at standard temperature and pressure (STP; 0°C and 1 atm), has a mass of 31.998 grams, and contains about 6.022 × 10 23 molecules of oxygen. Measuring one of these quantities allows the calculation of the others and this is frequently done in stoichiometry.
- The standard unit is called one mole of the substance. One mole of each of these different substances contains the same number of the particles (atoms, molecules, ions, electrons, protons, neutrons, etc). That number per mole has been worked by several different experimental methods and is found to be 6.0 × 10 23.
- A mole is the amount of pure substance containing the same number of chemical units as there are atoms in exactly 12 grams of carbon-12 (i.e., 6.023 X 1023).
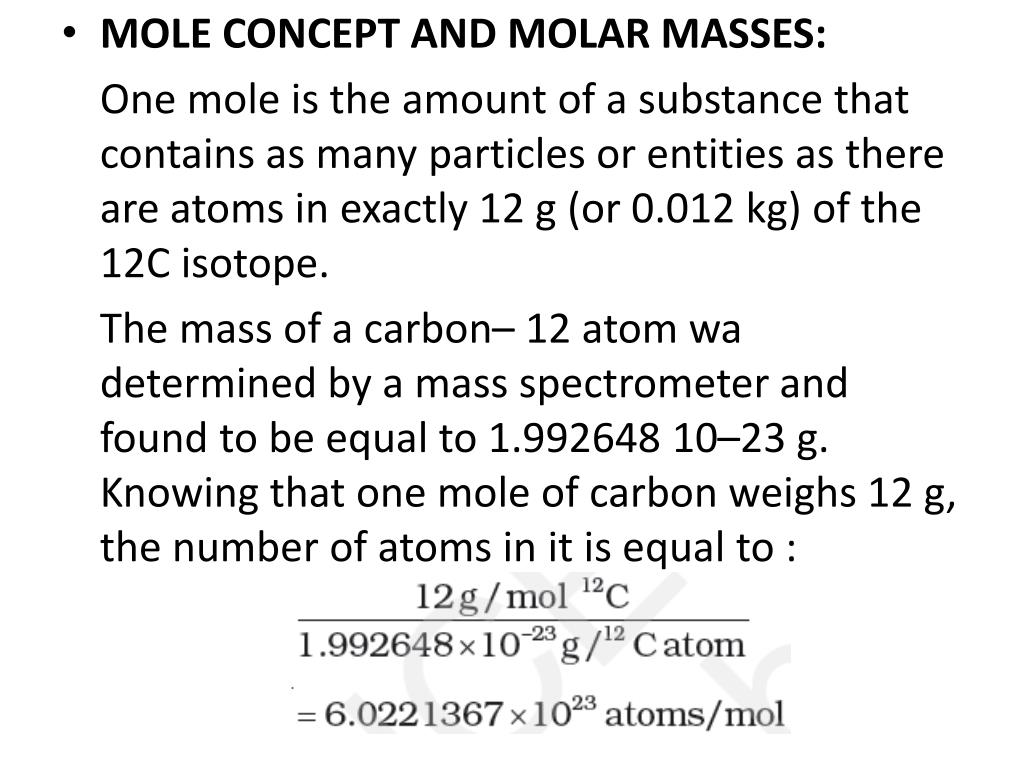
- The Mole The mole (abbreviated mol) is the SI measure of quantity of a “chemical entity,” such as atoms, electrons, or protons. It is defined as the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12. So, 1 mol contains 6.022×10 23 elementary entities of the substance.
- The mole (mol) is the amount of a substance that contains the same number of entities as there are atoms in exactly 12 g of carbon-12. One mole (1 mol) contains 6.022x10. Entities (to four significant figures). This number is called Avogadro’s number and is abbreviated as. The term “ entities ” refers to atoms, ions.
The mole is to the amount of substance (or chemical amount) as the gram is to mass. Like other units of the SI system, prefixes can be used with the mole, so it is permissible to refer to 0.001 mol as 1 mmol just as 0.001 g is equivalent to 1 mg.
Formal Definition
According to the National Institute of Standards and Technology (NIST), the Fourteenth Conférence Générale des Poids et Mesures established the definition of the mole in 1971.
The mole is the amount of a substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12; its symbol is 'mol.' When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
One Interpretation: A Specific Number of Particles
When a quantity of particles is to be described, mole is a grouping unit analogous to groupings such as pair, dozen, or gross, in that all of these words represent specific numbers of objects. The main differences between the mole and the other grouping units are the magnitude of the number represented and how that number is obtained. One mole is an amount of substance containing Avogadro's number of particles. Avogadro's number is equal to 602,214,199,000,000,000,000,000 or more simply, 6.02214199 × 10 23 .
Unlike pair, dozen, and gross, the exact number of particles in a mole cannot be counted. There are several reasons for this. First, the particles are too small and cannot be seen even with a microscope. Second, as naturally occurring carbon contains approximately 98.90% carbon-12, the sample would need to be purified to remove every atom of carbon-13 and carbon-14. Third, as the number of particles in a mole is tied to the mass of exactly 12 grams of carbon-12, a balance would need to be constructed that could determine if the sample was one atom over or under exactly 12 grams. If the first two requirements were met, it would take one million machines counting one million atoms each second more than 19,000 years to complete the task.
Obviously, if the number of particles in a mole cannot be counted, the value must be measured indirectly and with every measurement there is some degree of uncertainty. Therefore, the number of particles in a mole, Avogadro's constant ( N A ), can only be approximated through experimentation, and thus its reported values will vary slightly (at the tenth decimal place) based on the measurement method used. Most methods agree to four significant figures, so N A is generally said to equal 6.022 × 10 23 particles per mole, and this value is usually sufficient for solving textbook problems. Another key point is that the formal definition of a mole does not include a value for Avogadro's constant and this is probably due to the inherent uncertainty in its measurement. As for the difference between Avogadro's constant and Avogadro's number, they are numerically equivalent, but the former has the unit of mol −1 whereas the latter is a pure number with no unit.
A Second Interpretation: A Specific Mass
Atoms and molecules are incredibly small and even a tiny chemical sample contains an unimaginable number of them. Therefore, counting the number of atoms or molecules in a sample is impossible. The multiple interpretations of the mole allow us to bridge the gap between the submicroscopic world of atoms and molecules and the macroscopic world that we can observe.
One Mole Of A Substance Contains How Many Atoms Or Molecules
To determine the chemical amount of a sample, we use the substance's molar mass, the mass per mole of particles. We will use carbon-12 as an example because it is the standard for the formal definition of the mole. According to the definition, one mole of carbon-12 has a mass of exactly 12 grams. Consequently, the molar mass of carbon-12 is 12 g/mol. However, the molar mass for the element carbon is 12.011 g/mol. Why are they different? To answer that question, a few terms need to be clarified.
On the Periodic Table, you will notice that most of the atomic weights listed are not round numbers. The atomic weight is a weighted average of the atomic masses of an element's natural isotopes. For example, bromine has two natural isotopes with atomic masses of 79 u and 81 u. The unit u represents the atomic mass unit and is used in place of grams because the value would be inconveniently small. These two isotopes of bromine are present in nature in almost equal amounts, so the atomic weight of the element bromine is 79.904. (i.e., nearly 80, the arithmetic mean of 79 and 81). A similar situation exists for chlorine, but chlorine-35 is almost three times as abundant as chlorine-37, so the atomic weight of chlorine is 35.4527. Technically, atomic weights are ratios of the average atomic mass to the unit u and that is why they do not have units. Sometimes atomic weights are given the unit u , but this is not quite correct according to the International Union of Pure and Applied Chemistry (IUPAC).
To find the molar mass of an element or compound, determine the atomic, molecular, or formula weight and express that value as g/mol. For bromine and chlorine, the molar masses are 79.904 g/mol and 35.4527 g/mol, respectively. Sodium chloride (NaCl) has a formula weight of 58.443 (atomic weight of Na + atomic weight of Cl) and a molar mass of 58.443 g/mol. Formaldehyde (CH 2 O) has a molecular weight of 30.03 (atomic weight of C + 2 [atomic weight of H]) + atomic weight of O] and a molar mass of 30.03 g/mol.
The concept of molar mass enables chemists to measure the number of submicroscopic particles in a sample without counting them directly simply by determining the chemical amount of a sample. To find the chemical amount of a sample, chemists measure its mass and divide by its molar mass. Multiplying the chemical amount (in moles) by Avogadro's constant ( N A ) yields the number of particles present in the sample.
Occasionally, one encounters gram-atomic mass (GAM), gram-formula mass (GFM), and gram-molecular mass (GMM). These terms are functionally the same as molar mass. For example, the GAM of an element is the mass in grams of a sample containing N A atoms and is equal to the element's atomic weight expressed in grams. GFM and GMM are defined similarly. Other terms you may encounter are formula mass and molecular mass. Interpret these as formula weight and molecular weight, respectively, but with the units of u.
Avogadro's Hypothesis
Some people think that Amedeo Avogadro (1776–1856) determined the number of particles in a mole and that is why the quantity is known as Avogadro's number. In reality Avogadro built a theoretical foundation for determining accurate atomic and molecular masses. The concept of a mole did not even exist in Avogadro's time.
Much of Avogadro's work was based on that of Joseph-Louis Gay-Lussac (1778–1850). Gay-Lussac developed the law of combining volumes that states: 'In any chemical reaction involving gaseous substances the volumes of the various gases reacting or produced are in the ratios of small whole numbers.' (Masterton and Slowinski, 1977, p. 105) Avogadro reinterpreted Gay-Lussac's findings and proposed in 1811 that (1) some molecules were diatomic and (2) 'equal volumes of all gases at the same temperature and pressure contain the same number of molecules' (p. 40). The second proposal is what we refer to as Avogadro's hypothesis.
The hypothesis provided a simple method of determining relative molecular weights because equal volumes of two different gases at the same temperature and pressure contained the same number of particles, so the ratio of the masses of the gas samples must also be that of their particle masses. Unfortunately, Avogadro's hypothesis was largely ignored until Stanislao Cannizzaro (1826–1910) advocated using it to calculate relative atomic masses or atomic weights. Soon after the 1 st International Chemical Congress at Karlsrule in 1860, Cannizzaro's proposal was accepted and a scale of atomic weights was established.
To understand how Avogadro's hypothesis can be used to determine relative atomic and molecular masses, visualize two identical boxes with oranges in one and grapes in the other. The exact number of fruit in each box is not known, but you believe that there are equal numbers of fruit in each box (Avogadro's hypothesis). After subtracting the masses of the boxes, you have the masses of each fruit sample and can determine the mass ratio between the oranges and the grapes. By assuming that there are equal numbers of fruit in each box, you then know the average mass ratio between a grape and an orange, so in effect you have calculated their relative masses (atomic masses). If you chose either the grape or the orange as a standard, you could eventually determine a scale of relative masses for all fruit.
A Third Interpretation: A Specific Volume
One Mole Of A Substance Contains Dash Atoms Or Molecules
By extending Avogadro's hypothesis, there is a specific volume of gas that contains N A gas particles for a given temperature and pressure and that volume should be the same for all gases. For an ideal gas, the volume of one mole at STP (0°C and 1.000 atm) is 22.41 L, and several real gases (hydrogen, oxygen, and nitrogen) come very close to this value.
The Size of Avogadro's Number
To provide some idea of the enormity of Avogadro's number, consider some examples. Avogadro's number of water drops (twenty drops per mL) would fill a rectangular column of water 9.2 km (5.7 miles) by 9.2 km (5.7 miles) at the base and reaching to the moon at perigee (closest distance to Earth). Avogadro's number of water drops would cover the all of the land in the United States to a depth of roughly 3.3 km (about 2 miles). Avogadro's number of pennies placed in a rectangular stack roughly 6 meters by 6 meters at the base would stretch for about 9.4 × 10 12 km and extend outside our solar system. It would take light nearly a year to travel from one end of the stack to the other.
History
Long before the mole concept was developed, there existed the idea of chemical equivalency in that specific amounts of various substances could react in a similar manner and to the same extent with another substance. Note that the historical equivalent is not the same as its modern counterpart, which involves electric charge. Also, the historical equivalent is not the same as a mole, but the two concepts are related in that they both indicate that different masses of two substances can react with the same amount of another substance.
The idea of chemical equivalents was stated by Henry Cavendish in 1767, clarified by Jeremias Richter in 1795, and popularized by William Wollaston in 1814. Wollaston applied the concept to elements and defined it in such a way that one equivalent of an element corresponded to its atomic mass. Thus, when Wollaston's equivalent is expressed in grams, it is identical to a mole. It is not surprising then that the word 'mole' is derived from 'molekulargewicht' (German, meaning 'molecular weight') and was coined in 1901 or 1902.
SEE ALSO Avogadro, Amedeo ; Cannizzaro, Stanislao ; Cavendish, Henry ; Gay-Lussac, Joseph-Louis .
Nathan J. Barrows
Bibliography

Atkins, Peter, and Jones, Loretta (2002). Chemical Principles , 2nd edition. New York: W. H. Freeman and Company.
Lide, David R., ed. (2000). The CRC Handbook of Chemistry & Physics , 81st edition. New York: CRC Press.
Masterton, William L., and Slowinski, Emil J. (1977). Chemical Principles , 4th edition. Philadelphia: W. B. Saunders Company.

One Mole Of A Substance Contains


Internet Resources
One Mole Of A Substance Contains Atoms Or Molecules
National Institute of Standards and Technology. 'Unit of Amount of Substance (Mole).' Available from http://www.nist.gov .

Comments are closed.