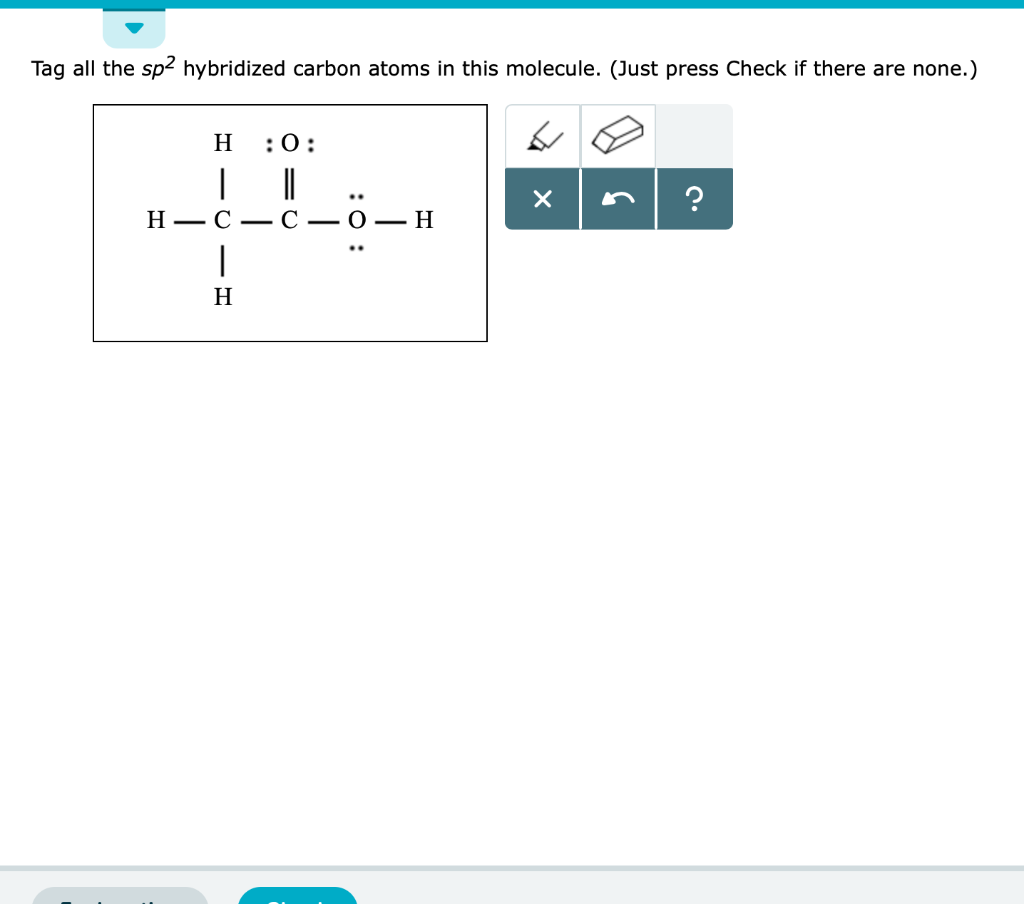
What is an sp2 hybridized carbon atom?
It means some sort of carbon-carbon double bond. Sp3 is single bonds, sp2 is double bonds, and sp is triple bonds. In ethylene, the carbon-carbon sigma bond is formed by overlap of one sp2 orbital (red) on each carbon atom. The carbon-hydrogen sigma bonds are formed by overlap of a carbon sp2 orbital (red) with a hydrogen 1 s orbital (blue). Overlap of one pz orbital (black) on each carbon forms the carbon-carbon pi bond.
1 Answer
carbon with

Explanation:
An example of carbon with
Carbon is tetravalent (forms 4 bond) and the ground state electron configuration cannot explain its valency since there's only 2 unpaired electron (left image below).
Therefore, one of the electron in 2s will be promoted to the empty 2pz orbital (middle image below).
In order to form the 3 sigma bonds in ethene, one 2s and two of the 2p orbitals will mix to form three
Sp2 Carbon Vs Sp3 Carbon
Note that the three
Sp2 Carbon Atoms
Related topic
Sp2 Carbons
Related questions

Comments are closed.