Atomic Number of Bromine is 35.
Chemical symbol for Bromine is Br. Number of protons in Bromine is 35. Atomic weight of Bromine is 79.904 u or g/mol. Melting point of Bromine is -7,3 °C and its the boiling point is 58,8 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearBromine Number Of Paired Electrons
About Bromine
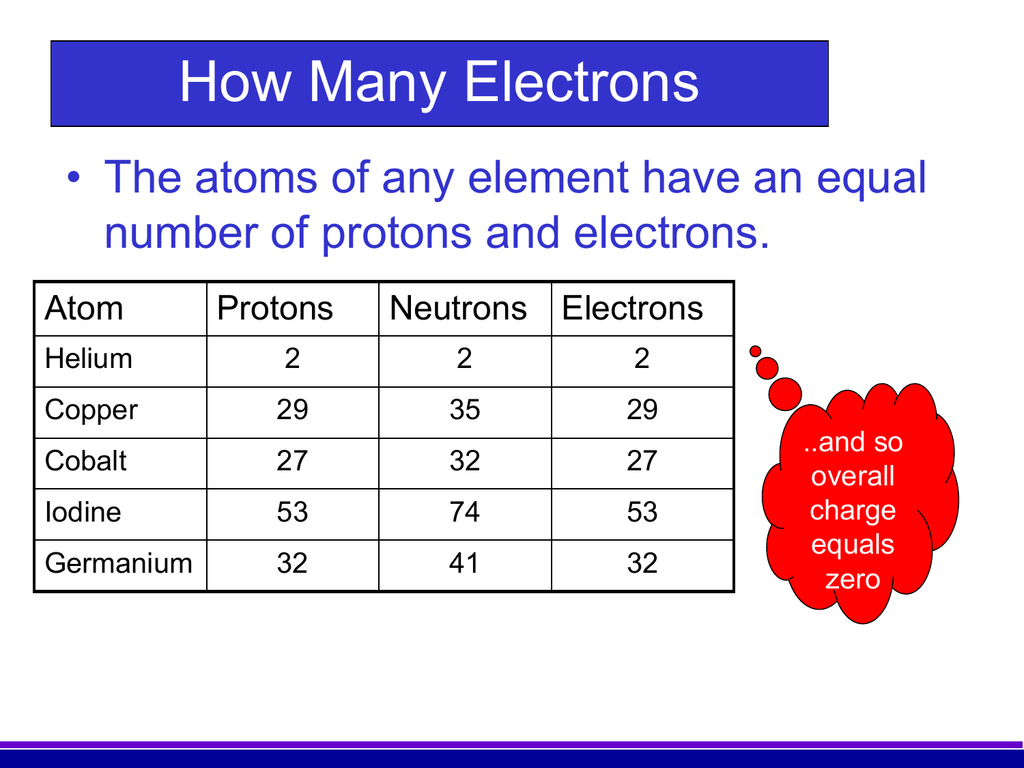
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Bromine is 35. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. In the case of Bromine the abbreviated electron configuration is Ar 3d10 4s2 4p5. Nevertheless, check the complete configuration and other interesting facts about Bromine that most people don't know.
Bromine is a toxic oily liquid of intense red color, known for its strong unpleasant smell. That is why its name comes from a Greek word meaning stench. This chemical element exists in our cells in the form of bromide, but in large doses it has very strong irritating and toxic properties so it should be avoided. Bromine belongs to the group of halogens. In nature, it can be found in some deposits in the soils, which are in abundance in North America and China, as well as in the Dead Sea. This chemical element is used in chemical industry for producing insecticides, pesticides, sedatives, and other chemicals. The compounds of this chemical element are added to various materials to reduce their flammability.
Uses of Bromine
Bromine, a reddish-brown element with symbol Br, is used in making dyes, in pharmaceuticals, in flame-retardants, in medicines, and in agricultural chemicals. Hydrogen bromide, a colorless compound with the formula HBr, is used as a catalyst in organic chemistry. It is also important in the production of inorganic and organic bromine alloys. Tetrabromoethane (C2H2Br4) and Bromoform (CHBr3) are used as liquids in gauges. Silver bromide, a compound with the formula AgBr, is mainly used in photographic films. Bromine monochloride, a compound with formula BrCl, is employed in analytical chemistry and industrial cooling water systems.
Compounds with Bromine
- HBr: Hydrogen bromide
- AgBr: Silver bromide
- BrN3: Bromine azide
- BrCl: Bromine monochloride
- BrF: Bromine monofluoride
- BrSCN: Bromine thiocyanate
- BrCN: Cyanogen bromide
- BrF3: Bromine trifluoride
- BrF5: Bromine pentafluoride
- HOBrO: Bromous acid
- HOBrO2: Bromic acid
- HOBrO3: Perbromic acid
- HOBr: Hypobromous acid
- BrOBrO3: Bromine perbromate
- C2H4Br2: Ethylene bromide
- C2H2Br4: Tetrabromoethane
- CHBr3: Bromoform
- KBrO3: Potassium bromate
- CBrClF2: Bromochlorodifluoromethane
- CBrF3: Bromotrifluoromethane
- CH2BrCl: Bromochloromethane
Properties of Bromine Element
| Atomic Number (Z) | 35 |
|---|---|
| Atomic Symbol | Br |
| Group | 17 |
| Period | 4 |
| Atomic Weight | 79.904 u |
| Density | 3.122 g/cm3 |
| Melting Point (K) | 265.8 K |
| Melting Point (℃) | -7,3 °C |
| Boiling Point (K) | 332 K |
| Boiling Point (℃) | 58,8 °C |
| Heat Capacity | 0.474 J/g · K |
| Abundance | 2.4 mg/kg |
| State at STP | Liquid |
| Occurrence | Primordial |
| Description | Halogen |
| Electronegativity (Pauling) χ | 2.96 |
| Ionization Energy (eV) | 11.81381 |
| Atomic Radius | 115pm |
| Covalent Radius | 114pm |
| Van der Waals Radius | 185 |
| Valence Electrons | 7 |
| Year of Discovery | 1826 |
| Discoverer | Balard |
What is the Boiling Point of Bromine?
Bromine boiling point is 58,8 °C. Boiling point of Bromine in Kelvin is 332 K.
What is the Melting Point of Bromine?
Bromine melting point is -7,3 °C. Melting point of Bromine in Kelvin is 265.8 K.
How Abundant is Bromine?
Abundant value of Bromine is 2.4 mg/kg.
What is the State of Bromine at Standard Temperature and Pressure (STP)?

State of Bromine is Liquid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Bromine Discovered?
Bromine Number Of Atoms
Bromine was discovered in 1826.
Bromine Number Of Electrons

Comments are closed.